Table of Contents
AQA | Unit 1 | Chemistry 1
Page 1 | Atoms, periodic table, chemical reactions
Page 2 | Limestone and Building Materials
Page 3 | Metal and their uses
Page 4 | Crude oil and fuels
Page 5 | Other useful substances from crude oil
Page 6 | Plant oils and their uses
Page 7 | Changes in the earth and its atmosphere
Page 1 | Structure and Bonding
Page 3 | Rates of Reactions
Page 4 | Exothermic and Endothermic Reaction
Page 5 | Acids, Bases and Salts
Page 6 | Electrolysis
AQA | Unit 3 | Chemistry 3
Page 1 | The periodic table
Page 2 | Water
Page 3 | Calculating and explaining energy change
Page 4 | Further analysis and quantitative chemistry
Page 5 | The production of ammonia
Page 6 | Alcohols, carboxylic acids and esters
Atomic structure, analysis and quantitative chemistry
Learning objectives:
To learn the relative masses of atoms can be used to calculate how much to react and how much we can produce
Atomic structure
The mass of an atom is relative to the number of protons and neutrons that are present in the nucleus. Each atom consists of a nucleus,
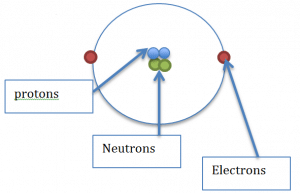
which contains the protons and the neutrons,
they then have electrons which exist on the outer energy levels.
Relative masses of subatomic particles
Protons and neutrons have a relative mass of 1 whereas electrons have a very small relative mass, which is equivalent of 1/1836
Mass number and atomic number
The mass number of an atom is the total number of protons and neutrons it contains.
The atomic number of an atom is the number of protons it contains
Therefore the mass number of an atom is never smaller the the atomic number
Isotopes
Elements will all have the same number of protons, but some elements will have different number of neutrons. Therefore this highlights that the atomic number will be the same where as the mass number will be different.
So atoms of the same element with different number if neutrons but the same number of protons are called isotopes
Chemical symbols
When looking at symbols in a periodic table the top number will shoe the mass number and the bottom the atomic number
So for example carbon
12
6 C this tells us that there are 6 protons, and as the mass number consists of protons and neutrons, to work out the number of neutrons, you follow this calculation
so in this case it will be 12 – 6 = 6
therefore carbon has 6 protons and 6 neutrons
Relative formula mass
The relative formula mass is found by adding together the relative atomic mass(otherwise known as mass number) of all the atoms in the compound.
The symbol for relative formula mass is Mr and the symbol for relative atomic mass is Ar
Therefore when working out the Mr in exams, Ar will be given in a table.
Example 1
Workout the Mr for CO2
So the Ar for carbon is 12 and the Ar for oxygen is 16
So 12+16+16 = 44
Moles
The relative formula mass of a substance can be shown in grams, this is referred to as the moles of a substance
Example 1
One mole of carbon dioxide has a mass of 28g
Therefore 14g will be equivalent to 0.5 moles and 56g will be equivalent to 2 moles
When doing mole calculations it is best to use this triangle

Analyzing substances
Chemists can use a number of methods to analyze substances and compounds.
Paper chromatography
Paper chromatography is used to analyze colored substances, paper chromatography works by dissolving the liquid, so they travel up the paper
This way of analyzing substances can be used for colored pigments and food additives.
Gas chromatography
Gas chromatography is a mixture of compounds to be separated.
⦁ The sample is dissolved in a liquid, then injected into of the machine
⦁ An unreactive gas, such as nitrogen carries the sample through the machine
⦁ The different substances within the sample travels through the machine at different speeds and are then separated
Mass spectrometry
Quantitative chemistry
In chemistry, candidates should be able to calculate the masses of reactant and products, percentage composition by mass, percentage yields, and empirical formulas.
Percentage composition
Percentage composition is a way to illustrate the different proportions of elements in a compound
You should be able to work out the percentage by mass of an element in a compound
To work out percentage composition follows the steps below
Example
The formula for sodium hydroxide is NaOH, work out the percentage by mass of oxygen
⦁ work out the Mr of the compound, using the Ar provided
⦁ so for NaOH it is , 23+16+1 = 40
⦁ take the Ar of oxygen and divide it by the total Mr.
⦁ 16/40 = 0.4
⦁ multiply by 100 to get a percentage – 0;4 x 100 = 40%
Percentage yield
Mass is never lost or gained in chemical reactions, so whatever we start with in the reactants, the same amount of mass will be present in the products
Using this simple principle it lets you calculate the theoretical mass of a product, however in reality this is not always possible, as some product may be lost, or some reactions may not go to completion
Yield can be defined in two different ways
⦁ Theoretical yield – this is the maximum mass of a product in a reaction (using the above principle)
⦁ Actual yield – this is the mass of a product when you actually carry out the reaction
Percentage yield is the ratio of actual yield compared to the maximum theoretical mass
Therefore the total equation is
Reversible Reactions
Many reactions are known as reversible, this means that reactions can go in both direction not just one
When writing a reversible reaction, scientist use this symbol
The symbol means that the reactant can break down the from the products, but also the products can react to form the reactant again
Reacting masses calculations
Example 1
CaCO3 Ca) + CO2
If we have 50g of CaCO3 how much CaO can we make?
First work of the Mr of the two compounds
CaCO3 is 40 + 12 +16 + 16 +16 = 100
CaO is 40 +16 = 56
This means the ration of the two is 100:56, so we can equate this to 100g of CaCO3 and 56g of CaO
However in the question we only have 50g, so we will only get half the amount of CaO as well which is 28g
For further help use ratios
The original ratio was 100:56
However in the question we only have 56grams
So 100 : 56
2 2
50 : 28
Empirical formula
The information given from reacting masses can be used to calculate the formula of a compound
Example
If 3.2g of sulfur reacts with oxygen to produce 6.4g of sulphur oxide, what is the formula of the oxide
Use these steps to calculate the formula
⦁ Find the masses – sulfur 3.2 and oxygen 3.2
⦁ Find the Ar of the elements – sulfur is 32 and oxygen is 16
⦁ Divide the mass by the Ar – 3.2/32 = 0.1 and 3.2/16 = 0.2
⦁ find the ratio – the ratio is 0.1:1.2 which is 1:2
⦁ so the final ratio is SO2
GCSE Chemistry
Structures and Bonding
Home | Chemistry | Structures and Bonding
Structure and Bonding
Learning objectives:
To learn how the arrangement of electrons in atoms can be used to explain what happens when elements react.
Ionic bonding
Ionic bonding occurs between non-metals and metals. Ionic bonding is the strong electrostatic force of attraction that is present between oppositely charged ions
Ions are electrically charged particles that are formed when an atom loses or gains an electron. The electron leaves from the highest energy level, which is furthest away from the nucleus.
Metal atoms lose electrons and become positively charged electrons Non-metal gains electrons and become negatively charged electrons
The elements in-group 1,2 and 3 will lose electrons based on the group number, for example aluminum will lose 3 electrons, as is it in-group 3
The elements in-group 5,6 and 7 will gain electrons based on the group they are in minus 8, for example oxygen in-group 6 will gain 2 electrons.
Drawing ions
When drawing ions, they are drawn in a specific way, as illustrated below
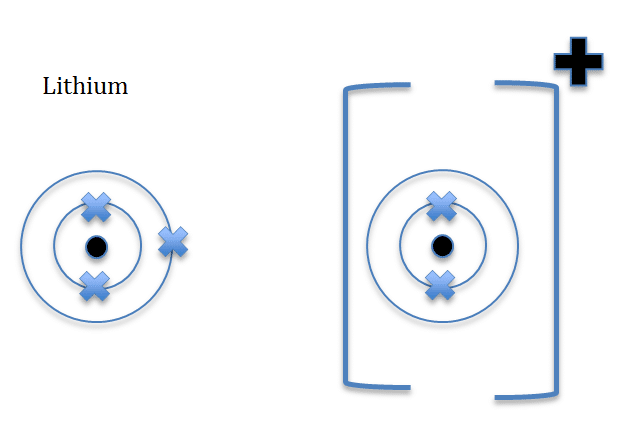
Lithium is in-group one, and is a metal and therefore will lose one electron and become a positive ion, Li+ is formed.
These set of diagrams will illustrate negative ions formed by the non metal elements
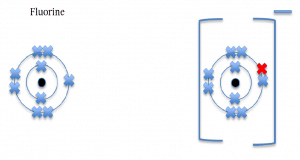
Fluorine, a group 7 element, will gain one electron and form a negative ion.
When metal react with non metals, an ionic compound is formed. This is an electrostatic force of attraction that is present between oppositely charged ions.
For example, if you take the two elements mentioned above.
Lithium + fluorine lithium fluoride
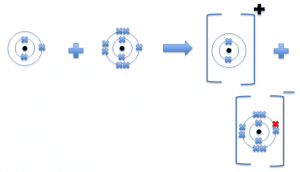
Ionic compounds
When non-metals and metals react they form ionic compounds. Strong electrostatic forces of attraction and the ions hold the ions together from a regular lattice structure.
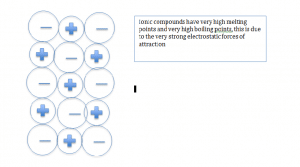
Formulae of ionic compounds
Ionic compounds are represented by a formula, which helps to highlight the atoms in the compound For example for lithium fluoride, it will be written as LiF. For compounds, which have more than one element attached can be written with brackets. For example iron hydroxide can be written as Fe(OH)3. This formula illustrates one iron atom joined with three oxygen and hydrogen atoms.
When constructing new formulas, we have to take into account the ionic number of the element. For example lithium has a number of +1 and fluorine has a number of -1, therefore we only need one of each to create the formula. In order to form the formula, the two numbers need to cancel each other’s out. So another example, when making the formula for magnesium chloride, magnesium is 2+ and chlorine is a 1-, therefore you would need two chlorines to cancel out the one magnesium, thus creating a MgCl2
Provided below is a list of positive ions and negative ions
Postive ions
| Name | Formula |
|---|---|
| Ammonium | NH4+ |
| Magnesium | Mg2+ |
| Hydrogen | H+ |
| Sodium | Na+ |
| Calcium | Ca2+ |
| Iron | Fe2+ or Fe3+ |
| Aluminium | Al3+ |
Negative ions
| Name | Formula |
|---|---|
| Flouride | F- |
| Magnesium | Mg2+ |
| Hydroxide | OH- |
| Nitrare | NO3- |
| Carbonate | CO3- |
| Sulfate | (SO4)2- |
| Sulfide | S2- |
| Oxide | O2- |
Covalent bonding
A covalent bond is a very strong bond between two non-metals. It is a shared pair of electrons between the two atoms
The number of lone electrons in its outer shell determines the number of covalent bonds an atom can make.
Covalent bonds are strong, a lot of energy is needed to break them, substances with covalent bonds have low melting an boiling point, for example water.
There is a quick way to work out how many covalent bonds an element can form. The number of covalent bonds an element can form is equivalent to 8 minus the group number .
So for example for oxygen, it is in group 6, so 8-6 = 2.
Drawing covalent bonds
Covalent bonds can be simply represented by a line between the two elements.
Examples of this can be seen below

Dot and cross diagrams
Covalent bonds are represented by dot and cross diagrams.
The simplest one is hydrogen or H2.

When drawing dot and cross diagrams for compounds you need to make sure all the cross over points has one cross and one dot.
Structure properties and uses
Compounds have different properties based on the bonding they undergo.
Simple covalent
Simple covalent bonds are formed between nonmetals, each bond consists of a shred pair of electrons.
Although the covalent bonds are really hard to break down and require a lot of energy, the intermolecular forces which are present within the molecules re broken down very easily which gives these molecules low melting and boiling points.
They do not conduct electricity because they do not have any free electrons to conduct electricity
Macromolecules
Macromolecules have giant covalent structures. This means there are many non metal atoms which are joined to adjacent atoms by covalent bonds. Their atoms are arranged in giant lattices.
These structures have very high letting points, due to the many strong covalent bonds that are present. An example of this is graphite or diamond
Diamond
Diamond is formed out of many carbon atoms, which are joined on to four other carbon atoms by strong covalent bonds, this ensures that diamond is a very hard substance and has a very high boiling point. However it does not conduct electricity, as there are no free electrons present.
Graphite
Graphite is another substance that is formed from carbon atoms, however graphite is formed In a different way to diamond. Graphite has layers of carbon sheets that can slide over each other. This illustrates why graphite is much softer than diamond. However graphite can conduct electricity as it has free electrons present within the layers of the carbon sheets
Silica
Silica is found is sand and has a similar structure to diamond, however it consists of silicon and oxygen atoms instead of carbon. Silica also has a very high melting point due to the many covalent bonds between the atoms.
Ionic compounds
Ionic compounds form when non-metal and metal ions react.
Ionic bonds are very strong, so a lot of energy is needed to break them; they have very high melting points and boiling points
Ionic compounds can conduct electricity when they are dissolved in water or dissolved, as the ions are free to move and carry and charge. However when they are in the solid state they are in a lattice form. So the ions are in a fixed position and cannot move to carry the charge.
Metals
When metals react with metals they form a metallic bond. This is when the positive metal ions are attracted to the electrons in the outer shell of the opposite ion. These electrons are de-localized meaning they are free to move. This ensures that a strong bond remains between the ions.
This is illustrated below

Metals are very good conductors of heat and electricity as they have free electrons which are free to move.
Metals are also malleable as the metals can slie over each other, due to the regular shape they are in.
Alloys
An alloy is a mixture of one or two elements. Usually alloys are a mixture of two or more metals.



