Table of Contents
AQA | Unit 1 | Chemistry 1
Page 1 | Atoms, periodic table, chemical reactions
Page 2 | Limestone and Building Materials
Page 3 | Metal and their uses
Page 4 | Crude oil and fuels
Page 5 | Other useful substances from crude oil
Page 6 | Plant oils and their uses
Page 7 | Changes in the earth and its atmosphere
AQA | Unit 2 | Chemistry 2
Page 1 | Structure and Bonding
Page 2 | Atomic structure, analysis and quantitative chemistry
Page 3 | Rates of Reactions
Page 4 | Exothermic and Endothermic Reaction
Page 5 | Acids, Bases and Salts
Page 6 | Electrolysis
AQA | Unit 3 | Chemistry 3
Page 1 | The periodic table
Page 2 | Water
Page 3 | Calculating and explaining energy change
Page 4 | Further analysis and quantitative chemistry
Page 5 | The production of ammonia
Page 6 | Alcohols, carboxylic acids and esters
Exothermic and endothermic reaction
Learning objectives:
To learn about energy changes in exothermic and endothermic reactions how the arrangement of electrons
Exothermic reactions
When a chemical reaction occurs, energy s either taken in or given out
Exothermic reactions transfer energy to the surrounding in the form of heat. This is usually indicated by a rise in temperature on a thermometer.
Most common exothermic reactions are combustion, oxidation or neutralization reactions
In terms of bonds, energy is releases when new bonds form, bond making is an exothermic process.
Endothermic reactions
These reactions will take in energy from the surroundings, therefore when measuring with a thermometer the temperature will go down. Examples of endothermic reactions are electrolysis and thermal decomposition reactions
In terms of bonds, energy is absorbed to break bonds so bond breaking is an exothermic process.
Energy diagrams
These diagrams show the different levels of energy in the reactants and the products. The bigger the difference the bigger the energy change. Energy diagrams help to illustrate whether a reaction is endothermic or exothermic
This is illustrated below
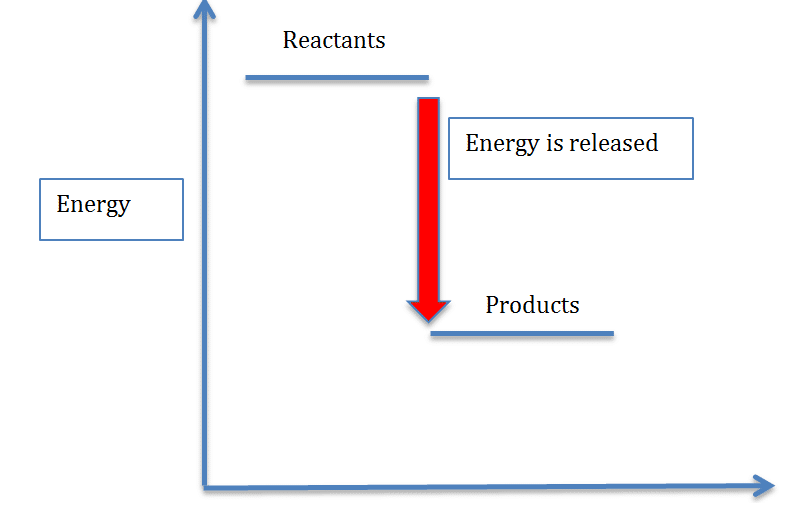
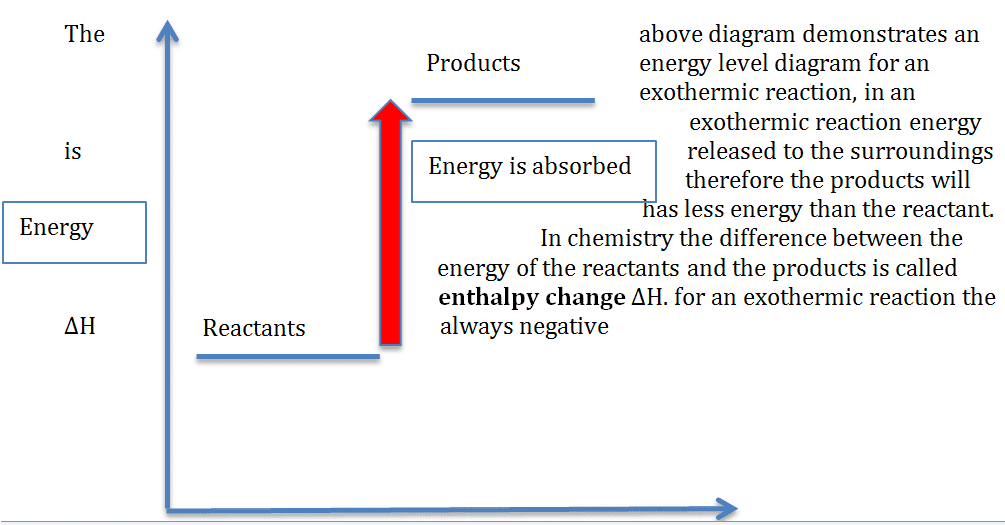
In an endothermic reaction, energy is absorbed so the products will always have more energy than the reactants, therefore the H is always positive.
Bond energy calculations
You can calculate the bond energy by measuring the total energy of the reactants and the total energy in the products; to calculate the change in energy you simply follow this equation
Worked example
H –H +Cl-Cl 2 x (H-Cl)
| Bond | Bond Energy |
|---|---|
| H-H | 436 |
| CL-CL | 243 |
| H-CL | 432 |
Energy of reactants = 436 + 243 = 679
Energy of products = 2 x 432 = 864
Energy change = reactants – products = 679 – 864 = -185
This shows that the reaction was exothermic

