Table of Contents
AQA | Unit 1 | Chemistry 1
Page 1 | Atoms, periodic table, chemical reactions
Page 2 | Limestone and Building Materials
Page 3 | Metal and their uses
Page 4 | Crude oil and fuels
Page 5 | Other useful substances from crude oil
Page 6 | Plant oils and their uses
Page 7 | Changes in the earth and its atmosphere
AQA | Unit 2 | Chemistry 2
Page 1 | Structure and Bonding
Page 2 | Atomic structure, analysis and quantitative chemistry
Page 3 | Rates of Reactions
Page 4 | Exothermic and Endothermic Reaction
Page 5 | Acids, Bases and Salts
Page 6 | Electrolysis
AQA | Unit 3 | Chemistry 3
Page 1 | The periodic table
Page 2 | Water
Page 3 | Calculating and explaining energy change
Page 4 | Further analysis and quantitative chemistry
Page 5 | The production of ammonia
Page 6 | Alcohols, carboxylic acids and esters
Structure and Bonding
Learning objectives:
To learn how the arrangement of electrons in atoms can be used to explain what happens when elements react.
Ionic bonding
Ionic bonding occurs between non-metals and metals. Ionic bonding is the strong electrostatic force of attraction that is present between oppositely charged ions
Ions are electrically charged particles that are formed when an atom loses or gains an electron. The electron leaves from the highest energy level, which is furthest away from the nucleus.
Metal atoms lose electrons and become positively charged electrons Non-metal gains electrons and become negatively charged electrons
The elements in-group 1,2 and 3 will lose electrons based on the group number, for
The elements in-group 5,6 and 7 will gain electrons based on the group they are in minus 8, for
Drawing ions
When drawing ions, they are drawn in a specific way, as illustrated below
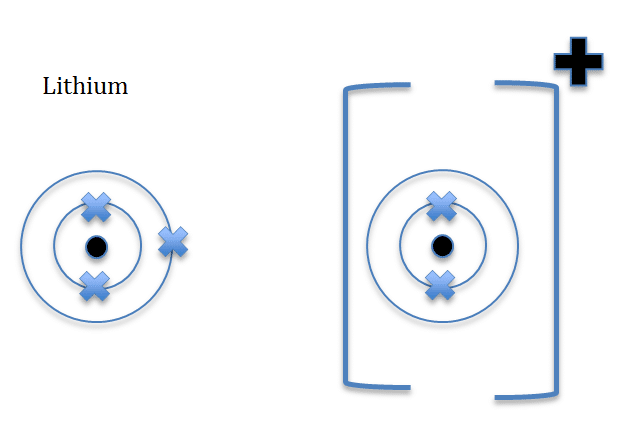
Lithium is in-group one, and is a metal and therefore will lose one electron and become a positive ion, Li+ is formed.
These set of diagrams will illustrate negative ions formed by the
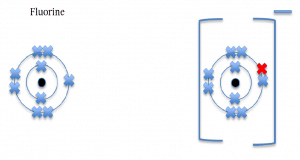
Fluorine, a group 7 element, will gain one electron and form a negative ion.
When metal
For example, if you take the two elements mentioned above.
Lithium + fluorine lithium fluoride
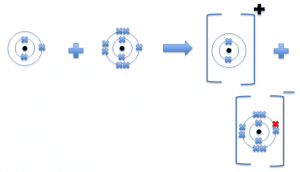
Ionic compounds
When non-metals and metals react they form ionic compounds. Strong electrostatic forces of attraction and the ions hold the ions together from a regular lattice structure.
Formulae of ionic compounds
Ionic compounds are represented by a formula, which helps to highlight the atoms in the compound For example for lithium fluoride, it will be written as LiF. For compounds, which have more than one element attached can be written with brackets. For
When constructing new formulas, we have to take into account the ionic number of the element. For
Provided below is a list of positive ions and negative ions
Postive ions
| Name | Formula |
|---|---|
| Ammonium | NH4+ |
| Magnesium | Mg2+ |
| Hydrogen | H+ |
| Sodium | Na+ |
| Calcium | Ca2+ |
| Iron | Fe2+ or Fe3+ |
| Aluminium | Al3+ |
Negative
| Name | Formula |
|---|---|
| Flouride | F- |
| Magnesium | Mg2+ |
| Hydroxide | OH- |
| NO3- | |
| Carbonate | CO3- |
| Sulfate | (SO4)2- |
| Sulfide | S2- |
| Oxide | O2- |
Covalent
The number of lone electrons in its outer shell determines the number of covalent bonds an atom can make.
Covalent bonds are strong, a lot of energy is needed to break them, substances with covalent bonds have low melting an boiling point, for
There is a quick way to work out how many covalent bonds an element can form. The number of covalent bonds an element can form is equivalent to 8 minus the group
So for example for oxygen, it is in group 6, so 8-6 = 2.
Drawing covalent bonds
Covalent bonds can be simply represented by a line between the two elements.
Examples of this can be seen below

Dot and cross diagrams
Covalent bonds are represented by dot and cross diagrams.
The simplest one is hydrogen or H2.

When drawing dot and cross diagrams for compounds you need to make sure all the
Structure properties and
Simple covalent
Simple covalent bonds are formed between nonmetals, each bond consists of a
Although the covalent bonds are really hard to break down and require a lot of energy, the intermolecular forces which are present within the molecules
They do not conduct electricity because they do not have any free electrons to conduct electricity
Macromolecules
Macromolecules have giant covalent structures. This means there are many
These structures have very high letting points, due to the many strong covalent bonds that are present. An example of this is graphite or
Diamond
Diamond is formed out of many carbon atoms, which are joined on to four other carbon atoms by strong covalent bonds, this ensures that diamond is a very hard substance and has a very high boiling point.
Graphite
Graphite is another substance that is formed from carbon atoms,
Silica
Silica is found is sand and has a similar structure to diamond,
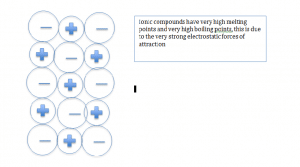
Ionic compounds
Ionic compounds form when non-metal and metal ions react.
Ionic bonds are very strong, so a lot of energy is needed to break them; they have very high melting points and boiling points
Ionic compounds can conduct electricity when they are dissolved in water or dissolved, as the ions are free to move and carry and charge.
Metals
When metals react with metals they form a metallic bond. This is when the positive metal ions are attracted to the electrons in the outer shell of the opposite ion. These electrons are de-localized meaning they are free to move. This ensures that a strong bond remains between the ions.
This is illustrated below

Metals are very good conductors of heat and electricity as they have free electrons which are free to move.
Metals are also malleable as the metals can
Alloys
An alloy is a mixture of one or two elements.



